Author: Denis Avetisyan
Researchers are leveraging the power of machine learning to accurately forecast the behavior of actuators built from living muscle tissue and flexible materials.
![The system employed four distinct waveform patterns to deliver electrical stimulation via a portable device, inducing contraction within a biohybrid muscle actuator positioned between movable pillars-a configuration designed to translate modulated [latex] ramp [/latex] or [latex] staircase [/latex] patterns of stimulation into observable mechanical movement.](https://arxiv.org/html/2602.16330v1/fig_ric.png)
This review details the application of both static and dynamic machine learning models, including Long Short-Term Memory networks, to predict the performance of biohybrid actuators for soft robotics applications.
Despite the promise of biohybrid actuators for soft robotics, inherent biological variability complicates precise control and prediction of performance. This challenge is addressed in ‘Machine Learning Driven Prediction of the Behavior of Biohybrid Actuators’, which investigates the application of supervised learning to model muscle-based actuators composed of tissue anchored on flexible polymer pillars. The study demonstrates high predictive accuracy using both static models – achieving an [latex]R^2[/latex] of 0.9425 – and dynamic Long Short-Term Memory networks with an [latex]R^2[/latex] of 0.9956. Will these predictive capabilities pave the way for robustly adaptive control strategies and unlock the full potential of biohybrid robotic systems?
The Inevitable Drift: Reimagining Robotics with Biological Systems
Conventional robotics frequently depends on systems constructed from rigid metals and powered by sizable, often electrically demanding, actuators. This design philosophy, while providing strength and precision in controlled environments, inherently limits a robot’s ability to navigate unpredictable terrain or interact safely with delicate objects. The inflexibility of these materials poses a challenge for applications requiring adaptability – such as search and rescue, biomedical interventions, or even subtle manipulation tasks. Moreover, the weight and power requirements of bulky actuators can significantly reduce operational efficiency and restrict a robot’s range of motion, hindering its potential in dynamic and complex scenarios. Consequently, researchers are increasingly exploring alternative approaches to robotic design, seeking materials and actuation methods that prioritize compliance, energy efficiency, and inherent safety.
Biohybrid machines represent a paradigm shift in robotics, moving beyond conventional systems reliant on rigid components and often inefficient power sources. These innovative devices directly integrate living muscle tissue – frequently sourced from cardiac or skeletal muscle – to generate motion and force. This approach unlocks the potential for remarkably nuanced and adaptable movements, mirroring the dexterity and responsiveness of biological organisms. Importantly, biological actuators boast an inherent energy efficiency exceeding many traditional motors, as muscle contraction is a naturally optimized process. Researchers envision applications ranging from minimally invasive surgical tools and prosthetic limbs with more natural control, to soft robots capable of navigating complex environments and interacting safely with humans – all powered by the elegant simplicity of living muscle.
Achieving truly functional biohybrid machines demands a deep comprehension of living muscle tissue as an actuator-it’s not simply about connecting biology to mechanics. Researchers are actively investigating the complex interplay between electrical stimulation, cellular response, and resulting contractile force, striving to model and predict muscle behavior with greater accuracy. This involves characterizing the non-linear dynamics of muscle cells, accounting for factors like fatigue, temperature sensitivity, and nutrient availability. Developing robust predictive models is crucial for designing control systems that can reliably harness biological power, allowing for precise and repeatable movements and ultimately unlocking the potential for adaptable, energy-efficient robotic systems that mimic the finesse of natural organisms.
Cultivating the Engine: Bioengineered Muscle and Stimulus Control
The creation of functional biohybrid systems relies on the consistent generation of mechanical work, demanding the development of bioengineered muscle tissue with predictable contractile properties. Current approaches focus on in vitro cultivation of myotubes or myofibers, often utilizing extracellular matrices to provide structural support and promote differentiation. Achieving reliable force output requires careful control over cellular alignment, nutrient delivery, and waste removal within the culture environment. Furthermore, maintaining consistent contractile characteristics over extended periods-a key challenge for long-term applications-necessitates strategies to prevent cellular degradation and maintain metabolic viability. Tissue engineering techniques, including decellularization and recellularization, are also being explored to create more complex and robust muscle constructs with enhanced functionality.
Electrical stimulation initiates muscle contraction in biohybrid systems by depolarizing the muscle cell membrane, triggering a cascade of events leading to the interaction of actin and myosin filaments. Precise control of stimulation parameters – including pulse amplitude (typically measured in volts or milliamps), pulse width (duration of the electrical pulse, in milliseconds), and frequency (pulses per second, or Hertz) – is critical for achieving predictable and reliable actuator movement. Insufficient stimulation may fail to elicit a response, while excessive stimulation can lead to muscle fatigue, damage, or tetanic contraction. Furthermore, the waveform of the stimulation – whether square, sinusoidal, or more complex – influences the efficiency and type of muscle contraction. Optimization of these parameters is therefore essential for maximizing force output and ensuring long-term system viability.
Accurate quantification of force output from bioengineered muscle constructs is essential for system validation and model development. Force measurements, typically expressed in Newtons (N) or milliNewtons (mN), allow for direct assessment of contractile performance against known stimuli. This data confirms whether the biohybrid system meets pre-defined functional requirements and establishes a baseline for iterative design improvements. Furthermore, the resulting force-displacement curves, and the derived parameters such as maximum isometric force and contraction velocity, serve as critical inputs for computational models. These models enable in silico prediction of system behavior under varying conditions, optimization of stimulation parameters, and ultimately, the scaling of biohybrid actuator performance. Precise force characterization also facilitates comparison between different muscle constructs and stimulation protocols, promoting standardized evaluation and reproducibility.
![A Long Short-Term Memory (LSTM) network accurately forecasts the biphasic asymmetric charge-balanced time series data generated by pulse modulation between [latex]2 ext{ms}[/latex] and [latex]10 ext{ms}[/latex] at [latex]30 ext{Hz}[/latex].](https://arxiv.org/html/2602.16330v1/figure5a.png)
From Static Prediction to Dynamic Control: Modeling Biological Response
Initial characterization of biohybrid actuators frequently centers on static prediction, which establishes the maximum force output achievable under defined conditions. This approach utilizes machine learning algorithms to model the relationship between input parameters and peak force. Random Forest Regression has been employed for this purpose, serving as a baseline for evaluating more complex models. Static prediction is crucial for understanding the actuator’s capabilities and limitations, providing a fundamental performance metric before investigating dynamic control strategies. The results of static prediction are essential for defining operational boundaries and ensuring safe and reliable performance.
Machine learning models were utilized to statically predict the behavior of biohybrid actuators, demonstrating high accuracy as measured by an R2 score of 0.9425. This static prediction involved determining the maximum force exerted by the actuator under defined conditions. Both a Neural Network regressor and a Long Short-Term Memory (LSTM) network were employed for this purpose, with the achieved R2 score representing a significant improvement over previously utilized models; specifically, a 9.6% improvement compared to initial Neural Network models and a 20.4% improvement over Random Forest Regression techniques.
The static force prediction model, utilizing a Neural Network regressor, achieved an R2 score of 0.9425. This performance represents a quantifiable improvement over previously employed methods; specifically, a 9.6% increase in predictive accuracy compared to initial Neural Network implementations and a 20.4% improvement when benchmarked against Random Forest Regression models. This enhanced predictive capability allows for more precise characterization of the biohybrid actuator’s maximum force output under defined conditions, serving as a foundational step towards dynamic control strategies.
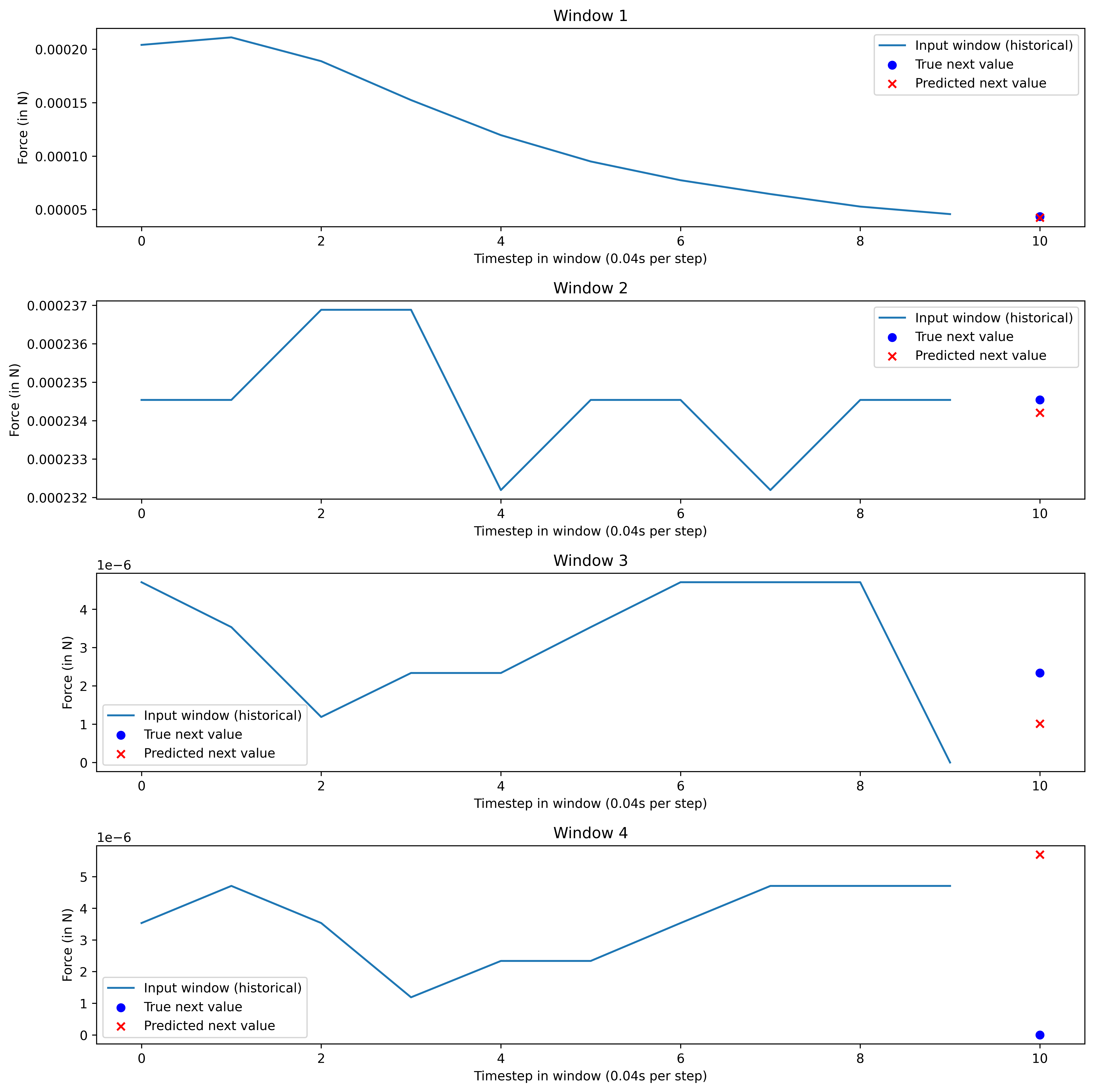
Achieving robust control of biohybrid actuators necessitates dynamic prediction, which involves forecasting the actuator’s force output as a function of time rather than simply determining maximum static force. This is accomplished through the application of recurrent neural networks, specifically Neural Network regressors and Long Short-Term Memory (LSTM) networks, trained on time-series data. These models learn the temporal dependencies inherent in actuator behavior, enabling prediction of force profiles beyond a single static measurement. The LSTM architecture, in particular, is well-suited to this task due to its capacity to retain information over extended sequences, improving the accuracy of dynamic force prediction compared to static prediction methods or simpler neural network models.
The Long Short-Term Memory (LSTM) model demonstrated high accuracy in predicting the time series force output of the biohybrid actuator, achieving an R2 score of 0.9956. This indicates that 99.56% of the variance in the observed force data is explained by the model’s predictions. Complementing this high R2 score, the model exhibited a Mean Squared Error (MSE) of 0.0013, representing the average squared difference between predicted and actual force values; a lower MSE indicates a better fit of the model to the data. These metrics collectively demonstrate the LSTM model’s capability to accurately forecast the actuator’s dynamic force profiles over time.
Time series forecasting techniques are integral to the performance of the Long Short-Term Memory (LSTM) model in predicting biohybrid actuator force profiles. These techniques facilitate the analysis of sequential data, allowing the LSTM to learn temporal dependencies and extrapolate future force values based on past behavior. Specifically, the LSTM model utilizes these techniques to process the time-dependent input data and generate accurate predictions, as demonstrated by its achieved R2 score of 0.9956 and Mean Squared Error of 0.0013. Without these forecasting methods, the LSTM’s ability to model the actuator’s dynamic force output would be significantly impaired, limiting its effectiveness in control applications.
The Virtual Replica: Proactive Control Through Digital Twins
The creation of a digital twin for biohybrid actuators represents a significant advancement in robotic control systems. This virtual counterpart isn’t merely a static model; it’s a dynamic, evolving representation built by fusing predictive algorithms with immediate feedback from force sensors. Real-time force measurements, captured as the biohybrid actuator operates, are continuously integrated into the model, refining its accuracy and allowing it to anticipate future performance. This constant calibration process enables the digital twin to accurately mirror the actuator’s behavior, providing a platform for testing control strategies and optimizing performance parameters without physically interacting with the biological component. Ultimately, this interconnectedness between the physical actuator and its virtual double facilitates a proactive approach to control, paving the way for more responsive and reliable biohybrid robotic systems.
Accurate prediction of a biohybrid actuator’s behavior hinges on a precise understanding of the baseline force generated by the living muscle tissue. This foundational force, representing the inherent contractile activity even without external stimuli, serves as the crucial calibration point for the digital twin’s predictive models. Without correctly characterizing this baseline – accounting for factors like muscle fiber composition, temperature, and nutrient availability – any attempt to forecast actuator response will be fundamentally flawed. Researchers meticulously measure this initial force through sensitive instrumentation, feeding the data into the virtual replica to refine its algorithms and ensure that simulations closely mirror real-world performance. This calibration process isn’t simply about achieving accuracy; it unlocks the potential for proactive control, allowing the system to anticipate and compensate for variations in muscle behavior, ultimately enhancing the actuator’s reliability and efficiency.
The creation of a functional digital twin for biohybrid actuators unlocks the potential for preemptive control strategies, moving beyond reactive adjustments to anticipate and optimize performance. By simulating the actuator’s behavior under various conditions, the virtual replica enables the implementation of control algorithms that proactively counteract potential issues – such as force fluctuations or instability – before they manifest in the physical system. This foresight dramatically improves the actuator’s efficiency, precision, and lifespan, while simultaneously opening doors to more complex robotic applications. Such capabilities are particularly crucial for tasks demanding delicate manipulation, adaptive locomotion, or sustained, reliable operation, ultimately paving the way for biohybrid actuators to be integrated into advanced prosthetics, minimally invasive surgical tools, and resilient, biomimetic robots.
![The neural network model accurately predicts force, as demonstrated by the strong correlation between actual and predicted values [latex] (R^2 = 0.95 [/latex]] and the near-zero mean residual distribution.](https://arxiv.org/html/2602.16330v1/figure2a.png)
The pursuit of predictive modeling, as demonstrated in characterizing biohybrid actuators, inherently acknowledges the transient nature of even the most innovative designs. This research, employing Long Short-Term Memory networks to forecast actuator behavior, isn’t about achieving permanent solutions, but rather about extending functional lifespan through informed adaptation. As David Hilbert noted, “We must be able to answer definite questions,” and this study answers the question of how to anticipate and manage the performance of these complex systems. Every abstraction-be it a machine learning model or a biological component-carries the weight of the past, demanding continuous refinement to preserve resilience against the inevitable decay inherent in all complex systems.
What’s Next?
The successful application of machine learning to biohybrid actuators, as demonstrated in this work, isn’t a destination but a versioning update. The models capture a present state, a snapshot of tissue behavior under specific conditions. However, biological systems are, fundamentally, systems of decay. Muscle tissue remodels, pillars fatigue, and the very definition of ‘actuation’ shifts over time. The arrow of time always points toward refactoring- toward models that don’t merely predict behavior, but anticipate its evolution.
A key limitation lies in the transferability of these predictive frameworks. Each instantiation of a biohybrid system is unique, a confluence of biological variation and fabrication imperfections. Future iterations should focus on developing models robust to these inherent uncertainties, perhaps through techniques like meta-learning or domain adaptation. The goal isn’t perfect prediction-that’s a static fantasy-but graceful degradation, an ability to maintain functionality even as the underlying components age.
Ultimately, the true measure of progress won’t be the accuracy of the models, but their capacity to inform design. Can these predictive tools facilitate the creation of biohybrid actuators that self-optimize, that adapt to their own changing properties? The pursuit of increasingly sophisticated algorithms is, of course, valuable. But the real challenge lies in integrating those algorithms with the inherent dynamism of living tissue, creating systems that age not with failure, but with a kind of biological wisdom.
Original article: https://arxiv.org/pdf/2602.16330.pdf
Contact the author: https://www.linkedin.com/in/avetisyan/
See also:
- MLBB x KOF Encore 2026: List of bingo patterns
- Overwatch Domina counters
- eFootball 2026 Jürgen Klopp Manager Guide: Best formations, instructions, and tactics
- 1xBet declared bankrupt in Dutch court
- eFootball 2026 Starter Set Gabriel Batistuta pack review
- Brawl Stars Brawlentines Community Event: Brawler Dates, Community goals, Voting, Rewards, and more
- Honkai: Star Rail Version 4.0 Phase One Character Banners: Who should you pull
- Gold Rate Forecast
- Lana Del Rey and swamp-guide husband Jeremy Dufrene are mobbed by fans as they leave their New York hotel after Fashion Week appearance
- Clash of Clans March 2026 update is bringing a new Hero, Village Helper, major changes to Gold Pass, and more
2026-02-19 16:22