Author: Denis Avetisyan
A new AI-powered system continuously analyzes wearable data to shift chronic care from reactive monitoring to proactive, personalized support.
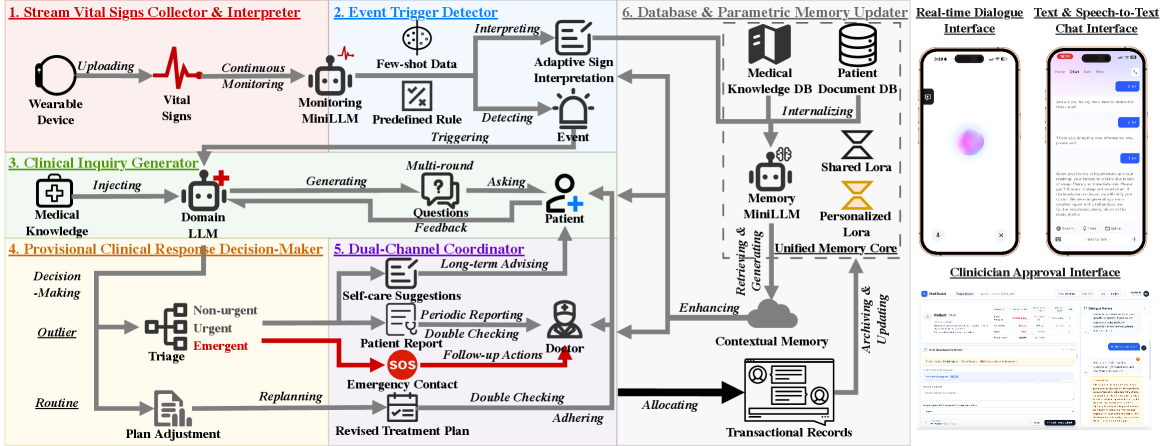
VitalDiagnosis integrates large language models with a dual-track framework and unified memory core for 24/7 vital sign analysis and chronic disease management.
Despite increasing strain on healthcare systems, effective chronic disease management often relies on passive monitoring and limited patient engagement. This paper introduces VitalDiagnosis: AI-Driven Ecosystem for 24/7 Vital Monitoring and Chronic Disease Management, an LLM-powered system designed to proactively address this challenge by integrating continuous data from wearable devices with a dual-track framework for personalized insights. By shifting from reactive alerts to interactive guidance, VitalDiagnosis aims to enhance patient self-management and reduce clinical workload. Could such an ecosystem represent a scalable pathway towards more cooperative and preventative healthcare paradigms?
The Inevitable Gaps in Episodic Care
Historically, managing chronic illnesses has depended on periodic evaluations – a patient visiting a physician every few months, or annually. This approach inherently misses the subtle, often gradual, shifts in a patient’s condition that occur between these appointments. Critical early indicators of decline – a slight change in gait suggesting worsening arthritis, a barely perceptible alteration in heart rate variability foreshadowing heart failure, or fluctuating blood glucose levels preceding a diabetic crisis – can go unnoticed, delaying intervention and potentially leading to more severe complications. This reactive model contrasts sharply with the potential for proactive healthcare, where continuous monitoring could reveal these early warning signs, enabling timely adjustments to treatment plans and improving patient outcomes before a crisis necessitates hospitalization or emergency care.
The proliferation of wearable sensors promises a revolution in healthcare, yet the sheer volume of generated data presents a significant analytical hurdle. These devices continuously collect physiological metrics – heart rate, activity levels, sleep patterns, and more – creating data streams far exceeding the capacity of traditional, episodic assessment. Transforming this constant flow into clinically relevant information demands sophisticated, real-time analysis techniques, including machine learning algorithms capable of identifying subtle anomalies and predicting potential health deteriorations. It’s not simply about collecting more data, but about intelligently processing it to discern meaningful signals from noise and delivering actionable insights to healthcare providers before a critical event occurs. This necessitates the development of systems that can not only handle the data’s velocity and variety but also integrate it seamlessly with existing electronic health records and clinical workflows.
Current healthcare infrastructure often falters when faced with the deluge of data produced by continuous monitoring devices. The sheer volume of information – heart rate, activity levels, sleep patterns, and more – quickly overwhelms traditional analytical methods, designed for intermittent snapshots rather than constant streams. Beyond quantity, the complexity arises from the interconnectedness of these data points and the need to discern meaningful signals from noise. This creates a critical bottleneck: while early warning signs of deterioration may be present in the data, existing systems lack the capacity for real-time processing and intelligent filtering necessary to alert clinicians to potential problems before they escalate, hindering proactive and timely intervention.
VitalDiagnosis: An Ecosystem, Not a Tool
VitalDiagnosis utilizes a Unified Memory Core to synthesize real-time data streams from wearable sensors with a patient’s longitudinal medical history. This core aggregates data including vital signs, activity levels, and sleep patterns, and integrates it with structured Electronic Health Record (EHR) data, unstructured clinical notes, and relevant medical knowledge graphs. The resulting contextualized patient profile allows for a more nuanced interpretation of current physiological states, enabling the system to differentiate between normal variations and potentially significant anomalies. This integration moves beyond simple threshold-based alerting by providing the LLMs with the necessary background to assess the clinical relevance of observed data points and facilitate informed decision-making.
The VitalDiagnosis Dual-Track Framework operates through two concurrent processes: proactive adherence monitoring and reactive anomaly triage. Adherence monitoring continuously assesses patient compliance with prescribed treatments and preventative care plans, utilizing data from wearable sensors and the Unified Memory Core to identify deviations from established baselines. Simultaneously, the anomaly triage component focuses on identifying and prioritizing unexpected data points indicative of potential health issues. This reactive track utilizes real-time data analysis and comparison against both individual patient history and population-level benchmarks to flag critical events requiring immediate attention. By operating these tracks in parallel, VitalDiagnosis aims to deliver comprehensive care by both preventing potential problems through proactive intervention and rapidly addressing emergent health concerns.
The VitalDiagnosis system utilizes a tiered Large Language Model (LLM) architecture comprised of three distinct models. A 4 billion parameter Memory MiniLLM stores and retrieves patient-specific historical data, providing contextual awareness. A 1.7 billion parameter Monitoring MiniLLM continuously analyzes incoming wearable data streams for deviations from established baselines. Finally, a 14 billion parameter Domain LLM serves as the primary analytical engine, leveraging information from both the Memory and Monitoring MiniLLMs to perform complex inquiries and generate insights, facilitating both proactive health management and reactive anomaly triage. This modular design allows for efficient resource allocation and specialized processing within the system.
Decoding the Signal from the Noise
The Event Trigger Detector employs a dual methodology for identifying anomalies in vital sign data. Rule-Based Thresholds utilize pre-defined limits for each vital sign; exceeding or falling below these limits immediately flags an event. Complementing this, Model-Based Inference leverages machine learning algorithms trained on historical data to establish baseline patterns for individual patients. Deviations from these personalized baselines, even within normal population ranges, are flagged as potential anomalies. This combined approach aims to maximize both sensitivity – detecting all true anomalies – and specificity – minimizing false positives arising from individual patient variability or normal physiological fluctuations.
The Monitoring MiniLLM functions as a clinical summarization tool, processing detected events from vital sign data and generating concise, clinically relevant narratives for healthcare providers. This LLM is specifically designed to translate raw data anomalies into understandable patient summaries, highlighting key findings and potential implications. The generated narratives prioritize brevity and clinical accuracy, enabling providers to quickly assess patient status and inform subsequent care decisions. Output focuses on presenting actionable information, avoiding verbose descriptions and focusing on clinically significant details derived from the event data.
The Clinical Inquiry Generator functions as an automated patient engagement tool, initiated by detected anomalies in vital sign data. Guided by the Domain LLM, this system formulates and delivers targeted questions to patients via various communication channels. The purpose of these inquiries is two-fold: to collect supplementary data that can refine the assessment of the detected event, and to corroborate initial findings by directly confirming symptoms or changes in condition with the patient. Responses are then processed and integrated into the patient’s record, providing clinicians with a more comprehensive and validated understanding of the situation before intervention.
The Illusion of Control: Augmenting, Not Replacing, Judgment
The system functions as a continuously evaluating clinical assistant, formulating proposed action plans by synthesizing a patient’s current events with their complete medical history. This Provisional Clinical Response Decision-Maker doesn’t simply react to immediate data; it actively interprets incoming physiological signals, lab results, and reported symptoms within the broader context of the individual’s past conditions, treatments, and responses. By leveraging this comprehensive understanding, the system can suggest interventions – from medication adjustments to further diagnostic tests – offering a dynamically tailored approach to patient care. This process isn’t about replacing clinical judgment, but rather augmenting it by providing a data-driven foundation for informed decision-making, ultimately aiming to improve both the speed and precision of medical responses.
The system’s proposed action plans aren’t enacted automatically; instead, they navigate a carefully constructed Tiered Approval Process designed to prioritize patient safety. This process categorizes recommendations based on potential risk, with lower-risk suggestions – such as minor medication adjustments – proceeding with automated implementation. However, any action deemed high-risk, including significant dosage changes or the introduction of new therapies, requires explicit review and approval by a qualified clinician. This human oversight acts as a crucial safeguard, ensuring that complex medical decisions aren’t made solely by the algorithm, but are instead informed by clinical judgment and a comprehensive understanding of the patient’s unique circumstances. The tiered approach balances the efficiency of automated support with the essential need for responsible, human-centered healthcare.
The system’s capacity for learning is central to its sustained effectiveness; the Database & Parametric Memory Updater functions as a continuous refinement engine. Through each patient interaction and subsequent outcome – whether positive, negative, or neutral – the system meticulously adjusts its internal parameters and expands its knowledge base. This isn’t simply data accumulation, but a dynamic process of pattern recognition and predictive modeling. Successful interventions reinforce certain associations, while unexpected results trigger a reassessment of contributing factors. Consequently, the system moves beyond initial programming, evolving its understanding of complex patient responses and ultimately delivering increasingly accurate and personalized recommendations over time. This iterative improvement minimizes reliance on static rules and maximizes adaptability to the inherent variability of individual patient needs.
Beyond Prediction: Fostering Clinical Literacy
VitalDiagnosis seeks to empower individuals facing chronic illnesses through enhanced clinical literacy, delivering tailored insights directly related to their specific health profiles. The system moves beyond generalized medical information by analyzing patient data – encompassing symptoms, lifestyle factors, and medical history – to generate personalized explanations of their conditions and treatment plans. This isn’t simply about presenting data; it’s about translating complex medical terminology into understandable language, fostering a more informed and engaged patient. By clarifying the ‘why’ behind their care, VitalDiagnosis aims to help individuals actively participate in managing their health, promoting adherence to treatment, and ultimately leading to better outcomes and a higher quality of life.
The VitalDiagnosis system achieves truly personalized chronic care through an innovative adaptive parametric memory, powered by Low-Rank Adaptation (LoRA). This technology allows the system to continuously refine its understanding of an individual patient’s condition without requiring extensive retraining of the core model. LoRA efficiently modifies a small subset of parameters within the larger model, effectively creating a unique “memory” of each patient’s responses to interventions and changes in their health status. As new data becomes available – from wearable sensors, patient-reported outcomes, or clinical visits – the system dynamically adjusts care pathways, optimizing treatment plans and preventative measures for maximum efficacy. This continuous learning loop ensures that the care received isn’t static, but rather evolves in tandem with the patient’s needs, representing a significant leap towards precision and proactive chronic disease management.
The convergence of personalized diagnostics and adaptive treatment pathways signals a fundamental change in how chronic diseases are addressed. Rather than reacting to crises, this integrated ecosystem prioritizes continuous monitoring and preemptive intervention, shifting the focus from episodic care to sustained health management. By leveraging technologies that learn and adapt to individual patient needs, the system aims to not only mitigate disease progression but also enhance overall well-being. This proactive approach has the potential to significantly reduce the economic burden associated with chronic conditions, lessening the strain on healthcare resources while simultaneously improving patient outcomes and quality of life through earlier detection and personalized strategies.
The pursuit of VitalDiagnosis, an ecosystem for continuous health oversight, echoes a fundamental truth about complex systems. It isn’t merely about assembling components, but nurturing a responsive, evolving whole. The system, much like a garden, requires constant attention and adaptation. As Blaise Pascal observed, “The belly is an ungovernable master.” This resonates with the article’s dual-track framework; acknowledging the inherent unpredictability of biological systems – the ‘belly’ of health – and building in mechanisms for graceful degradation and proactive engagement. The focus on a Unified Memory Core isn’t about achieving perfect recall, but enabling forgiveness between components when inevitable failures occur, fostering resilience through interconnectedness.
What Lies Ahead?
The architecture presented here, while aiming for proactive care, subtly encodes a belief in predictable decay. Each LoRA adapter, each unified memory core, is a temporary bulwark against the inevitable drift of physiological baselines and the evolving complexity of chronic conditions. The system anticipates failure modes it has been explicitly trained to recognize – a comforting illusion, perhaps, but one that will fracture when confronted with genuinely novel presentations. It is not a question of if the model will misinterpret a cascade of unforeseen variables, but when.
The true challenge isn’t simply processing more wearable data, or even refining the dual-track framework. It lies in acknowledging that ‘personalized support’ is a perpetually moving target. The ecosystem will demand constant recalibration, not through architectural improvements, but through an acceptance of inherent instability. The system’s success will be measured not by its predictive power, but by its graceful degradation – its ability to signal its own diminishing returns before a critical failure.
Future iterations will likely focus on explainability, on making the ‘black box’ more transparent. However, increased transparency won’t eliminate uncertainty; it will merely redistribute it. The goal shouldn’t be to solve chronic disease, but to build a system that can coexist with it, adapting and evolving alongside the patient-a symbiotic, rather than curative, approach. The fear, ultimately, is not of the technology failing, but of it succeeding too well, creating a brittle, over-optimized solution incapable of handling the messy reality of long-term health.
Original article: https://arxiv.org/pdf/2601.15798.pdf
Contact the author: https://www.linkedin.com/in/avetisyan/
See also:
- VCT Pacific 2026 talks finals venues, roadshows, and local talent
- EUR ILS PREDICTION
- Lily Allen and David Harbour ‘sell their New York townhouse for $7million – a $1million loss’ amid divorce battle
- Will Victoria Beckham get the last laugh after all? Posh Spice’s solo track shoots up the charts as social media campaign to get her to number one in ‘plot twist of the year’ gains momentum amid Brooklyn fallout
- Vanessa Williams hid her sexual abuse ordeal for decades because she knew her dad ‘could not have handled it’ and only revealed she’d been molested at 10 years old after he’d died
- Dec Donnelly admits he only lasted a week of dry January as his ‘feral’ children drove him to a glass of wine – as Ant McPartlin shares how his New Year’s resolution is inspired by young son Wilder
- Invincible Season 4’s 1st Look Reveals Villains With Thragg & 2 More
- SEGA Football Club Champions 2026 is now live, bringing management action to Android and iOS
- The five movies competing for an Oscar that has never been won before
- How to have the best Sunday in L.A., according to Bryan Fuller
2026-01-25 06:51